Our Story

Our Story
Gibson Oncology is focused on a portfolio of anti-cancer agents already in clinical trials that were discovered by and licensed from the National Institutes of Health (NIH) and Purdue University. The NIH has been committed enough to the potential importance of these new anti-cancer compounds that it has completely supported all work from discovery to conducting 5 clinical trials.
Gibson Oncology and its investors have had the benefit that all of this exciting and expensive work was done with non-dilutive financing for Gibson. It has spent its resources on ensuring the agents are available at pharmaceutical grade, are patented, and are protected by FDA filings with designations for specific new orphan drug and pediatric use. Gibson has also invented its own patented 2nd generation compounds, elucidated new and innovative anti-cancer mechanisms for its compounds, and is now poised to move into Phase II clinical trials.
Gibson’s agents are called the indenoisoquinolines (Indenos) and they are designated LMP744, LMP400, and LMP776. The NIH has been very excited about them because they are truly novel non-camptothecin topoisomerase-1 agents. They have features that markedly improve their effectiveness and safety over well-known older and frequently used drugs like irinotecan and topotecan. The Indenos form more stable ternary DNA complexes containing the Indeno, and Topo-1, and, importantly, are not subject to efflux pumps that cause the older cancer agents to stop working by being pumped out of the cancer cell.
This is what the NIH has said about these agents:
The indenoisoquinolines (Indenos) represent the first non-camptothecin Topo-1 inhibitors for the treatment of cancer”, and have unique anti-cancer properties.
All 3 Indenos display “Lethal Synergy with Olaparib”, the leading treatment for breast cancer under the name Lynparza.
The NIH has demonstrated that the Indenos and Olaparib can safely be given together in animal models.
The NIH has documented a key mechanism by which the Indenos work with a characteristic genetic (HRD) marker.
The NIH has developed a “molecular rational to do a Phase II trial” in BRACA breast cancer and said, in writing, that a phase II trial is warranted.
Although the current Phase I clinical trials are being done with IV administration, the NIH has demonstrated that the Indenos also can be orally administered in animal models of human disease.
The NIH had originally conducted four clinical trials on two of the Indenos but, when the canine veterinary trial showed a dramatic 78% response rate in canine lymphoma with the third agent, LMP 744, the NIH decided to focus on that agent and initiated the most recent clinical trial on recurrent solid tumors and lymphomas. The clinical trial is still ongoing but already has had patient responses*.
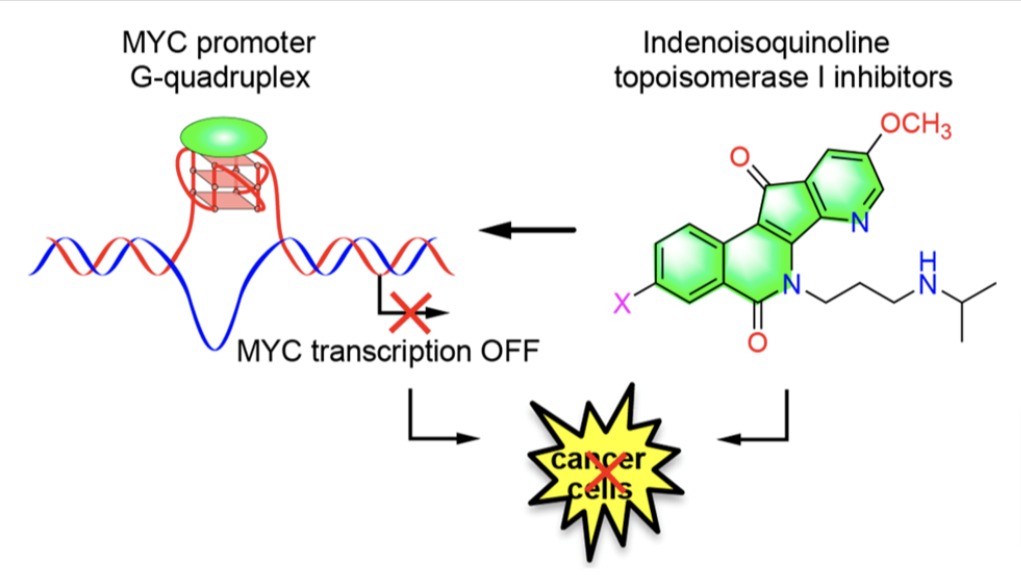
As this focus on LMP744 developed, Gibson Oncology discovered that several of our compounds, and particularly LMP744, had both Topo-I-mediated and C-MYC-mediated anticancer activity. The discovery of inhibition of C-MYC protein expression is very important because C-MYC is considered to be one of the most important causes of cancer, as
- C-MYC is aberrantly expressed in 70-80% of cancers, yet is still considered “undruggable” by any approved agent.
- C-MYC is one of the most important transcription factors that are involved in multiple types of cancer cell proliferation and chemoresistance.
- C-MYC when properly regulated causes tumors to regress.
- Previous attempts over decades to target C-MYC, a major oncogene involved in driving 80% of all tumors, have failed because of the peculiar characteristics of the shallow binding pockets on the C-MYC protein, which created the reputation of being an “undruggable” target. Gibson’s novel compounds were rationally designed to avoid this issue through a novel mechanism of action involving the stabilization of the G-quadruplex in the C-MYC promoter, resulting in inhibition of transcription of the C-MYC gene.
Gibson Oncology now has a truly unique agent (LMP744) that has both Topo-1-mediated and C-MYC-mediated activity. Furthermore, it is already approved for clinical trials, has responses in an NIH human clinical trial, and has an increasing number of important preclinical studies suggesting additional uses. The NIH has supported and completely funded this work and clearly believes in the potential of these compounds as important anti-cancer agents.
Reasons the NIH Believes Phase II Trials Are Indicated:
- LMP agents work effectively in tumors with homologous recombinant deficiency (HRD) including BRACA-1 and BRACA-2 (e.g., breast, ovarian) and high levels of Schlafen11 (SLFN11).
- LMP agents display “lethal synergy” with olaparib (Lynparza), a multi-billion dollar drug that is used frequently for breast cancer.
- All 3 Indenoisoquinolines were also synergistic with Olaparib, especially in HRD cells. Our results confirm a rationale for molecularly designed clinical trials with the Indenoisoquinolines as single agents and in combination with Olaparib.
- Irinotecan and topotecan are used to treat a variety of different cancers. However, they have limitations, including chemical instability and severe side effects. To overcome these limitations, we developed the clinical indenoisoquinolines: LMP400 (indotecan), LMP776 (indimitecan), and LMP744. The purpose of the study was to build the molecular rationale for Phase II Clinical Trials.
- As a result, Gibson Oncology is in the process of applying to the FDA for permission to do a Phase II trial in BRACA-1/-2 breast cancer.
PLANNED PHASE II CLINICAL TRIALS WTH THE INDENOS
PHASE II TRIAL IN BREAST CANCER IN PATIENTS WITH BRACA-1 AND BRACA-2 GENETIC DEFICIENCIES
While about 13% of women in the general population will develop breast cancer, 55%-72% of women who inherit a harmful BRACA-1 variant and 45%-69% of women who inherit a harmful BRACA-2 variant will develop breast cancer.
The BRACA -1 and BRACA- 2 variants are genetically inherited defects that tend to occur in specific population groups. Their genetic defect simultaneously occurs on both strands of the patient’s DNA and produces breast cancer and also other cancers like ovarian cancer.
The Indenos are already approved for clinical testing and have been tested in Phase I clinical trials in a wide range of recurrent solid tumors and lymphomas. To conduct Phase II trials, there needs to be scientific evidence for the FDA to approve trials for a specific indication.
The evidence cited above by the NIH clearly warrants a phase II trial in BRACA related breast cancer.
PHASE II TRIAL IN BRAIN METASTASES IN BRACA-1/-2 BREAST CANCER
“One of the most feared sequelae after a diagnosis of breast cancer is the development of metastases to the brain.” The reason is that “there are no US FDA-approved treatments specifically for breast cancer brain metastases.”
One of the key reasons is that there are few drugs that work on specific subtypes of breast cancer and also cross the blood-brain barrier.
There is a major need since the incidence of brain metastases ranges from 10% to nearly 50% in certain subtypes of breast cancer, and there is evidence that BRACA-related tumors have an increased incidence of brain metastases.
Gibson has shown that the Indenos cross the blood-brain barrier and others have shown that they also have lethal synergy with Olaparib. Treating brain tumor metastases of breast cancer is a logical next step.
PHASE II TRIAL IN GLIOBLASTOMA MULTIFORME (GBM)
Glioblastoma multiforme (GBM) is one of the most deadly and aggressive forms of cancer. It is generally well known to the public, despite the fact that GBM only occurs in about 10,000-15,000 people a year because it has caused the rapid and unanticipated death of prominent people. There is no cure and life expectancy is about 10-15 months on average.
A wide range of genetic and environmental factors like radiation have been discussed as risk factors. With increased ability to do rapid DNA sequencing, biopsies obtained from patients at surgery have shown several dozen genetic types of variation occur in these tumors.
When Gibson Oncology discovered that the Indenos had both Topo-1 and C-MYC activity and that, in addition, the agents cross the blood-brain barrier, the NIH became interested in testing the effect of these agents on GBM. To do this, they used known and type-specific genetic variations of cell cultures derived from the cells of patients with GBM tumors. They were able to demonstrate dramatic effectiveness of our agents, particularly LMP744. It dramatically slowed the growth of GBM cells in culture compared to control and at remarkably low levels of drug concentration.
As this was occurring, we were contacted by a number of major medical centers with programs in brain cancer who have asked us to provide our agents to them to conduct additional studies. Most of this work will be done under material transfer agreements in which Gibson Oncology provides the drugs, the medical centers do the research, and there are clear guidelines of intellectual property discovered in these studies.
Simultaneously, Gibson has begun discussions with the NIH about doing a Phase II study in patients who have tumors with the specific GBM cellular changes that that were associated with dramatic responses to our compounds in cell culture. These discussions ae ongoing with a plan hopefully to be worked out by fall of 2023.
Evidence shows that:
- LMP744 crosses the blood-brain barrier (BBB) in levels sufficient to produce concentrations in the brain that mimic the concentrations needed to impair metastatic tumor and GBM growth in cellular studies.
- LMP744 can potentially be used with PARP inhibitors to treat breast cancer patients with brain metastases. LMP synergy with PARP inhibitors (NIH quote) described above and the fact that LMP744 crosses the blood-brain barrier is the rationale for this statement.
MORE EVIDENCE
Most recently, there have been several developments that strengthen the potential role of the Indenos in GBM. Multiple publication now suggest PARP inhibitors for treatment of GBM and there is even a clinical study with encouraging result using Olaparib and Temazolamide. Clearly, given the favorable characteristics of the Indenos compared to Temazolamide, and the fact that our drugs provide a documented “Lethal Synergy” with Olaparib, a combined study of LMP 744 and Olaparib would be justified.
Our other agent, LMP 400, has many overlapping features with LMP 744 and appears to be a candidate for treating GBM as well.
We are also exploring other brain tumors such as pontine gliomas that might also demonstrate such effective responses to the Indenos.
ADDITIONAL INFORMATION
Gibson Oncology now has a second generation of newly invented and patented drugs, also with combined Topo-1 and C-MYC activity (the AZAs). These agents also show non-camptothecin Topo-1 effects like the Indenos, and Gibson has demonstrated that the AZAs inhibit C-MYC protein expression through stabilization of the G-quadraplex in the C-MYC promoter in multiple cancer cell lines. We already know that some of those AZAs cross the blood-brain barrier and inhibit GBM cellular growth in vitro. We are now entering in vivo trials in GBM animal models.
Gibson Oncology has a significant number of international patents on the Indenos and the new AZAs.
Gibson Oncology has filed and continues to file for FDA designations that protect Gibson’s rights to provide drugs in various orphan drug and pediatric indications.
*Additional information is available with an executed Confidential Disclosure Agreement.
